55502493
Kohlenstoff
In 10 interaktiven Modulen und in interaktiven Videos wird Wissen zu Kohlenstoff vermittelt und abgefragt.
Das Medium bietet H5P-Aufgaben an, die ohne zusätzliche Software verwendbar sind.
Durch interaktive Aufgabentypen wird das audiovisuelle und interaktive Lernen einfach.
Lernen macht jetzt Spaß!
Included Tasks
- I Was ist Kohlenstoff - Lückentext
- II Kohlenstoff im Alltag - Aufgaben mit Video
- III Kohlenstoffarten - interaktives Video
- IV Kohle und Kohlenstoff - interaktives Quiz
- V Kohlenstoff im Alltag - interaktive Aufgabe
- VI Summenformeln für Spezialisten - interaktive Flashkarten
- VII Strukturformeln und Summenformeln - interaktive Aufgabe
- VIII Kohlenstoff im Buchstabengitter - interaktive Aufgabe
- IX Säure-Basen-Eigenschaften - interaktive Aufgabe
- X Kohlenstoff- interaktive Aufgaben
Curriculum-centred and oriented towards educational standards
Matching
Carbohydrates
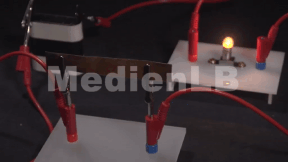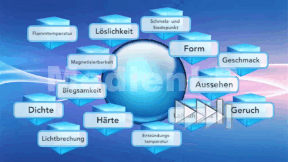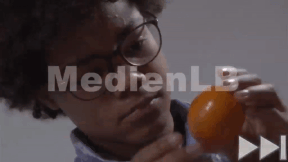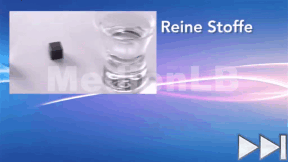
The term carbohydrate or saccharide is a collective name for all substances with the chemical formula Cn(H2O)n. Carbohydrates are the basis of nutrition. They are part of our diet as starch, glucose (grape sugar), fructose (fruit sugar), lactose (milk sugar) and saccharose (beet, cane or table sugar). Important suppliers of carbohydrates are potatoes and cereals such as rice, wheat, maize, millet, rye and oats. The various carbohydrates in our foods are introduced to the pupils. The characteristics of polysaccharides, disaccharides and monosaccharides are explained to them and in which foods these substances occur and how they are structured. In addition, the different origins of starch, starch degradation products, gelling agents as well as sugar alcohols in confectionery are dealt with. The DVD shows how various substances can be detected with the help of chemical processes. Together with the extensive accompanying material the DVD is ideally suited for use in the classroom.
Materials and Substances of Everyday Life
Hearing these words, you first think of the materials our clothing is made of. But all objects surrounding us in everyday life consist of one or several materials.
Fascination Lime
Many products used in everyday life are impossible without lime. These are, among others, glass, sugar, paper as well as pharmaceutical and cosmetic products. The raw material is also indispensable in the construction materials industry. Iron and steel producers need limestone, in environmental protection it is used, for example, for air cleaning and drinking water purification.