55503281
Kohlenhydrate: zuckersüße Chemie
In 10 interaktiven H5P-Modulen wird Wissen zum Thema Kohlenhydrate vermittelt und anschließend abgefragt.
Das Medium bietet H5P-Aufgaben an, die ohne zusätzliche Software verwendbar sind.
Durch interaktive Aufgabentypen wird das audiovisuelle und interaktive Lernen einfach.
Lernen macht jetzt Spaß!
Included Tasks
- I Nahrungsgrundlage: Kohlenhydrate - Aufgaben mit Video
- II Energiekreislauf - interaktive Aufgabe
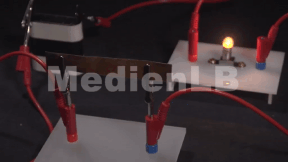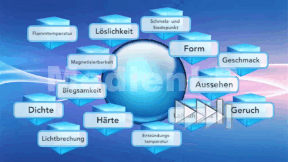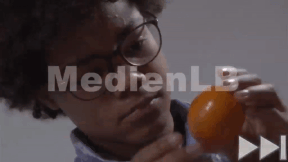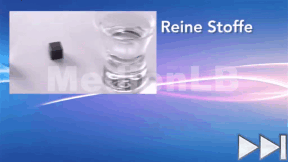
- III Zucker ist nicht gleich ZUCKER - interaktive Aufgabe
- IV Aufbau der Kohlenhydrate - Lückentext
- V Fehling-Probe - interaktives Video
- VI Einteilung der Kohlenhydrate - interaktive Aufgabe
- VII Einfachzucker: Monosaccharide - interaktive Aufgabe
- VIII Zweifachzucker: Disaccharide - interaktive Aufgabe
- IX Stärkenachweis - Video mit Aufgaben
- X Kohlenhydrate-Quiz - interaktive Aufgabe
Curriculum-centred and oriented towards educational standards
Matching
Halogens
The compounds of halogens are - with the exception of astatine - widespread, can be encountered in nature and are versatile substances. This fact is taken up on this DVD in order to teach the students the chemistry of the halogens by illustrating their special qualities and explaining the correlation of their structure with their chemical properties. In the first part, an overview of the element group of halogens lays emphasis on the common as well as on the distinguishing characteristics of fluorine, chlorine, bromine and iodine. In a second part, the specific properties of fluorine and chlorine are presented. This topic is linked to the students‘ everyday experience (fluorine as a protection against caries, chlorine as a disinfectant, etc.) on the one hand. On the other hand, the DVD presents carefully selected experiments. As a rule, they are of a kind that can only be realized with difficulty, or high expenditure in the chemistry classroom. With the help of these experiments, students are introduced to the chemistry of the halogens in a way that enables them to draw conclusions on the basis of their observations.